YU, development of “Diatom-mimicking micelle-based ultrafilm silica nanoparticle biocatalyst” N
No.7742803- Writer PR team
- Date : 2023.09.26 14:58
- Publication Date : 2023.09.14
- Views : 12366
Professor KIM Chang-seop’s research team developed “ultra-film silica nanoparticles to be used as a carbon reduction biocatalyst”
Increased utility of enzyme-based green chemistry technologies through improved mass transfer and stability
Published in the latest issue of Chemical Engineering Journal, an internationally renowned journal in the field of chemical engineering
[September 14, 2023]

'<From the left, YU Professor KIM Chang-seop, student LEE Ae-seol, and Dr. HEO Hye-ryeong>
The research team of Professor KIM Chang-seop (40) and Dr. Heo Hye-ryeong (36) of YU Department of Chemistry of YU (President CHOI Oe-chool) developed micelle-based ultrafilm silica nanoparticles to be used as a carbon reduction biocatalyst.
Enzymes have advantages such as high activity and selectivity, few by-products, and environmentally friendly conditions, but their use in industry is limited due to their low stability. However, Carbonic anhydrase (CA)*1 is a biocatalyst that hydrates carbon dioxide and is a protein that is receiving great attention in environmental industries as it can be used in carbon capture and sequestration (CCS) technology.
Recently, Professor KIM's research team developed a biomimetic enzyme encapsulation technology to increase the stability of carbonic anhydrase enzyme and increase its industrial use as a carbon reduction biocatalyst.The research team said, “With the technology developed this time, we can utilize the biosilicification reaction of diatoms to imitate the environment and structure within diatoms*2 and at the same time protect enzymes from external stress environments. Diatom-derived silica-forming peptides and carbonic anhydride Nanoparticles with a polar silica layer were developed using micelle*3 with enzymes immobilized on the surface as a template. We succeeded in increase of enzyme stability with the silica layer of the polar membrane and succeeded in minimization of mass transfer limitations.”
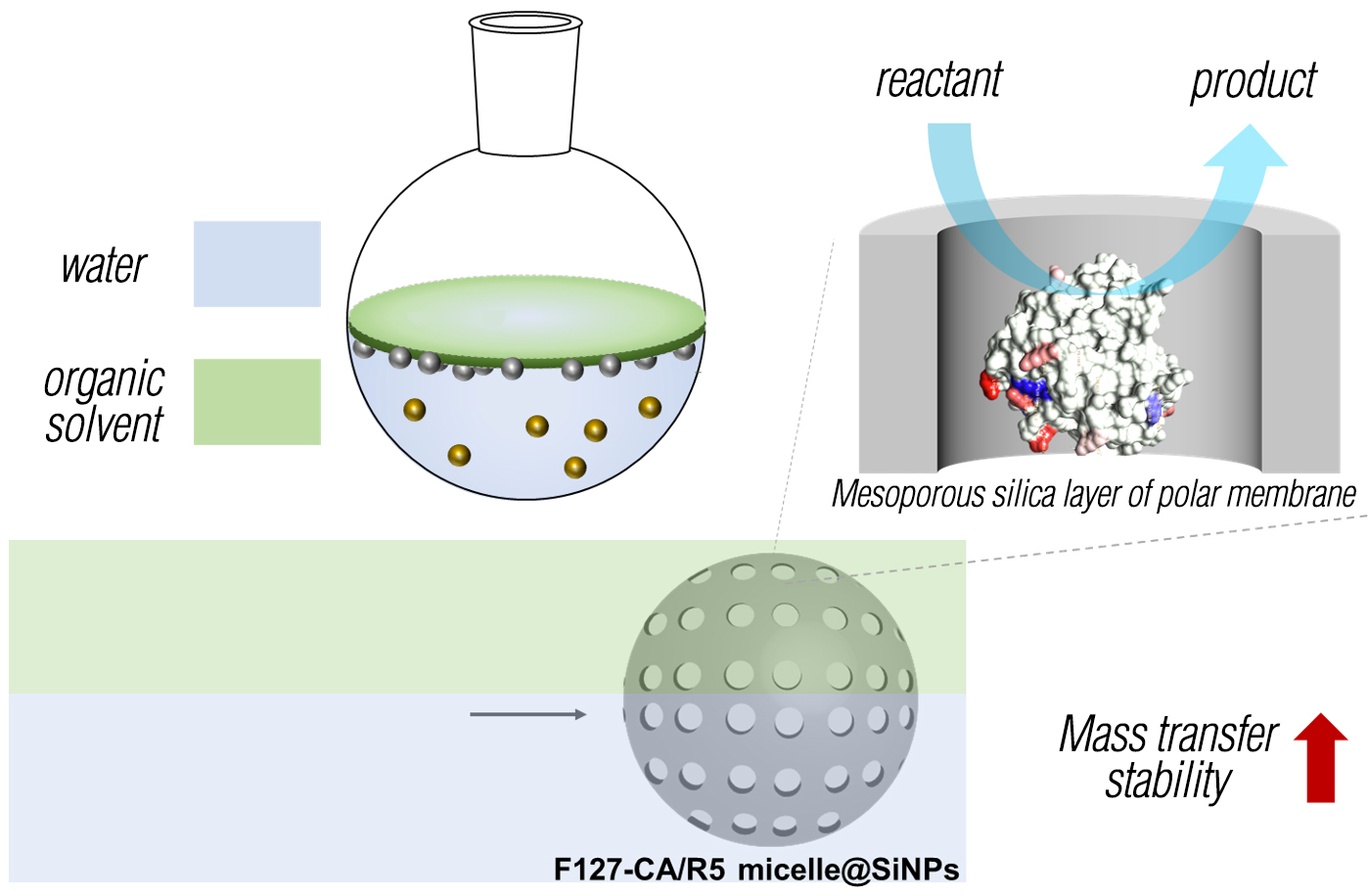
<Schematic diagram of a polar membrane mesoporous silica nanoparticle biocatalyst with high mass transfer efficiency and stability>
Professor KIM Chang-seop, who led the research team, said, “The micelle-based ultrafilm silica nanoparticle biocatalyst developed this time showed improved stability even under carbon capture and removal technology (CCS) reaction conditions. This shows the possibility that the biocatalyst developed through this can be used in an actual industrial environment. The utilization of enzyme-based technology can be increased, and there are no restrictions on applicable enzymes or biomolecules, so we plan to conduct research to apply it to biomedical engineering applications.”
LEE Ae-seol (30, Ph.D. completed) from Department of Biochemistry at YU Graduate School participated as the first author in this study and the research paper was published in the renowned international journal <Chemical Engineering Journal> in the chemical engineering field (impact factor (IF) 15.1, top tier). (within 5%) was pre-released online in the latest issue (dated October 1, 2023).This research was conducted with support from the New Researcher Support Project, the Sejong Science Fellowship Project and from the Project of Medical Research Center (MRC) of Leading Research Center supported by the Ministry of Information & Communication and National Research Foundation of Korea.
[Terminology]
*1 Carbonic Anhydrase (CA): A protein that reacts carbon dioxide and water to produce carbonate 10 million times faster than the natural reaction.
*2 Diatoms: Single-celled microorganisms that perform photosynthesis and are abundant in the sea, but are also plankton that live in some freshwater lakes.
*3 Micelles: Aggregates of surfactants such as soap gathered at a certain concentration or higher. A three-dimensional structure in which the water-hating (hydrophobic) part forms the nucleus and the water-loving (hydrophilic) part forms the surface in contact with water.